History...
A collaboration of undergraduate students, clinicians, and biomedical engineers undertook developing a therapy for vasospasm symptoms in patients with Raynaud’s phenomenon in 2017. Our journey began with a written request by a physician on behalf of his wife, seeking a non-pharmacologic therapeutic option for her Raynaud’s symptoms. We explored the pathophysiology of the disorder, and current therapy. We decided to pursue cellular signaling mechanisms of microvascular structures. Our collective experience in photonics, medical devices, and management of patients with this disorder proved beneficial.
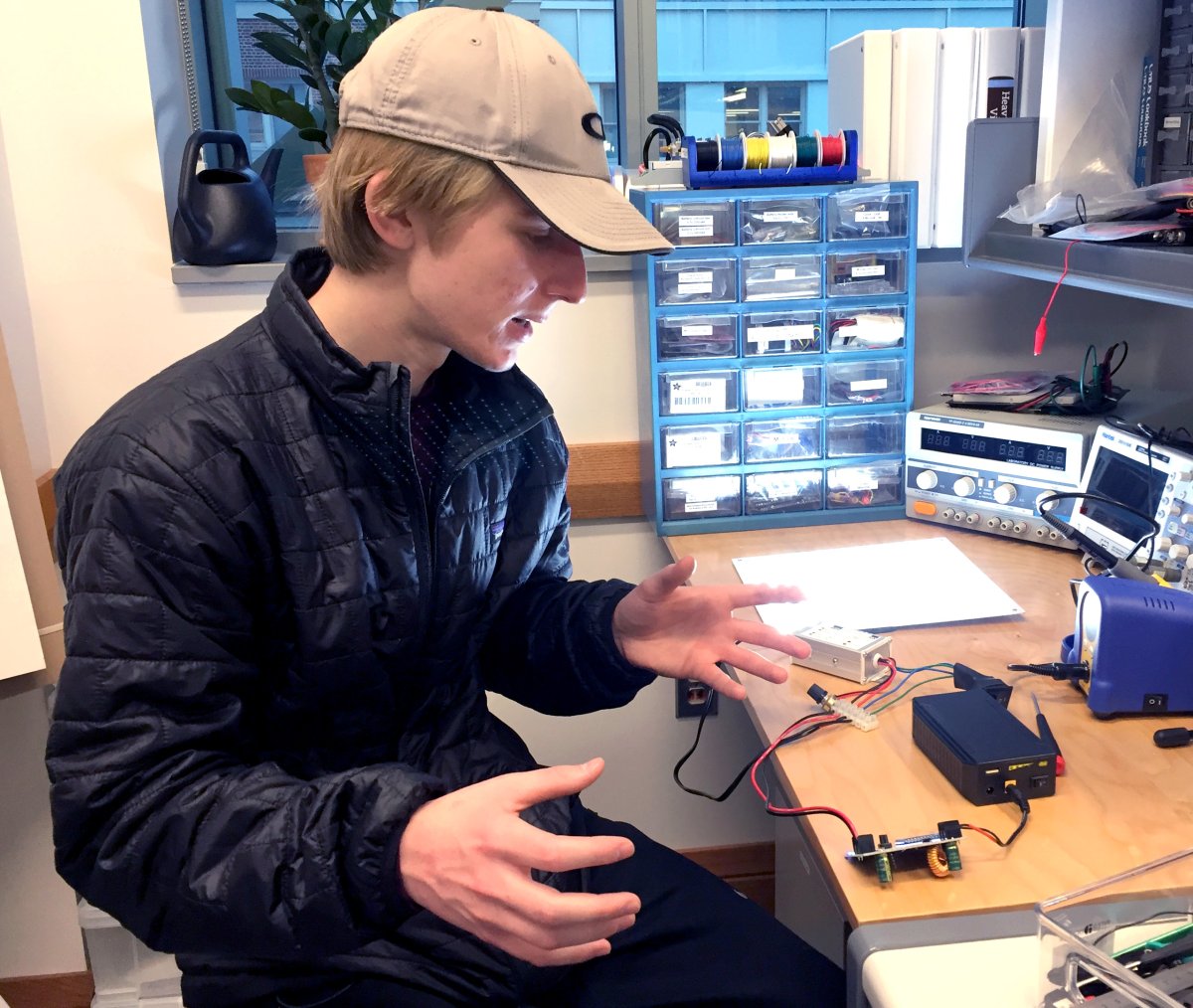
Among the challenges was creating a precision low-level light source at a specified light wavelength to modulate a signaling pathway of the microvascular structures. This is different than relatively high-energy consuming thermal-only techniques. The Phototherapy Device has evolved from a foam-board mockup, through three prototypes, to the present research tool. Hopefully, what we learned can be translated into low-energy consuming hand and feet garments, with embedded light sources for patient use.
Achievements...
On January 9, 2024, the authors and the University of Minnesota received a utility patent (U.S. 11,865,357) for the light-based treatment devices and methods.
On March 26, 2024 the project was published in the Annals of Biomedical Engineering, titled: An Experimental Phototherapy Device for Studying the Effects of Blue Light on Patients with Raynaud’s Phenomenon.
A clinical study was approved by the University of Minnesota Institutional Review Board. Once funded and underway, this will begin the development phase for a potential treatment option.
Phototherapy Device Study Instrument...

The final instrument was constructed from numerous engineering drawings, schematics and printed circuit board designs. It was the result of three previous prototypes, each testing a new and needed aspect of the required device. Both rapid prototyping and contemporary manufacturing techniques were utilized to produce a safe, accurate and precise study tool. Skills required to accomplish this were taught in Prof. Saliterman's Medical Device Prototyping (BMEN 2151) and Medical Device Practicum (BMEN 3151) courses.
Kushal Sehgal, James Kerber and Emily Wagner, Practice Session...

A clinical study protocol was approved by the University of Minnesota Institutional Review Board (IRB) in 2021. The orange eyewear blocks blue light and is helpful in conducting a double blinded crossover study.
Interview with Jennifer Brooks, Columnist in the Star Tribune...
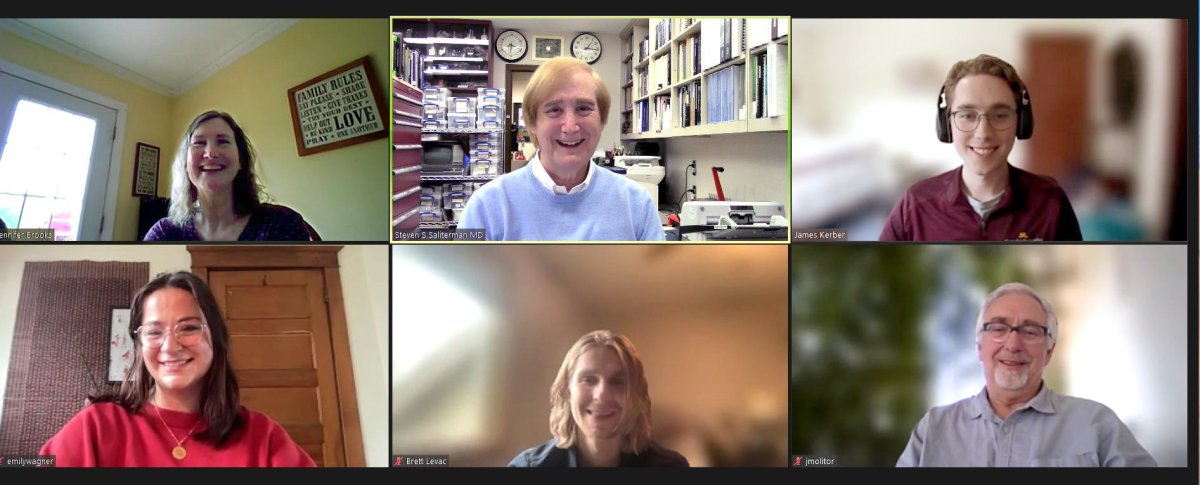
Top row: Jennifer Brooks, Steven Saliterman & James Kerber; and bottom row: Emily Wagner, Brett Levac & Jerry Molitor. Not present were Kushal Sehgal and Jennifer Chmura.
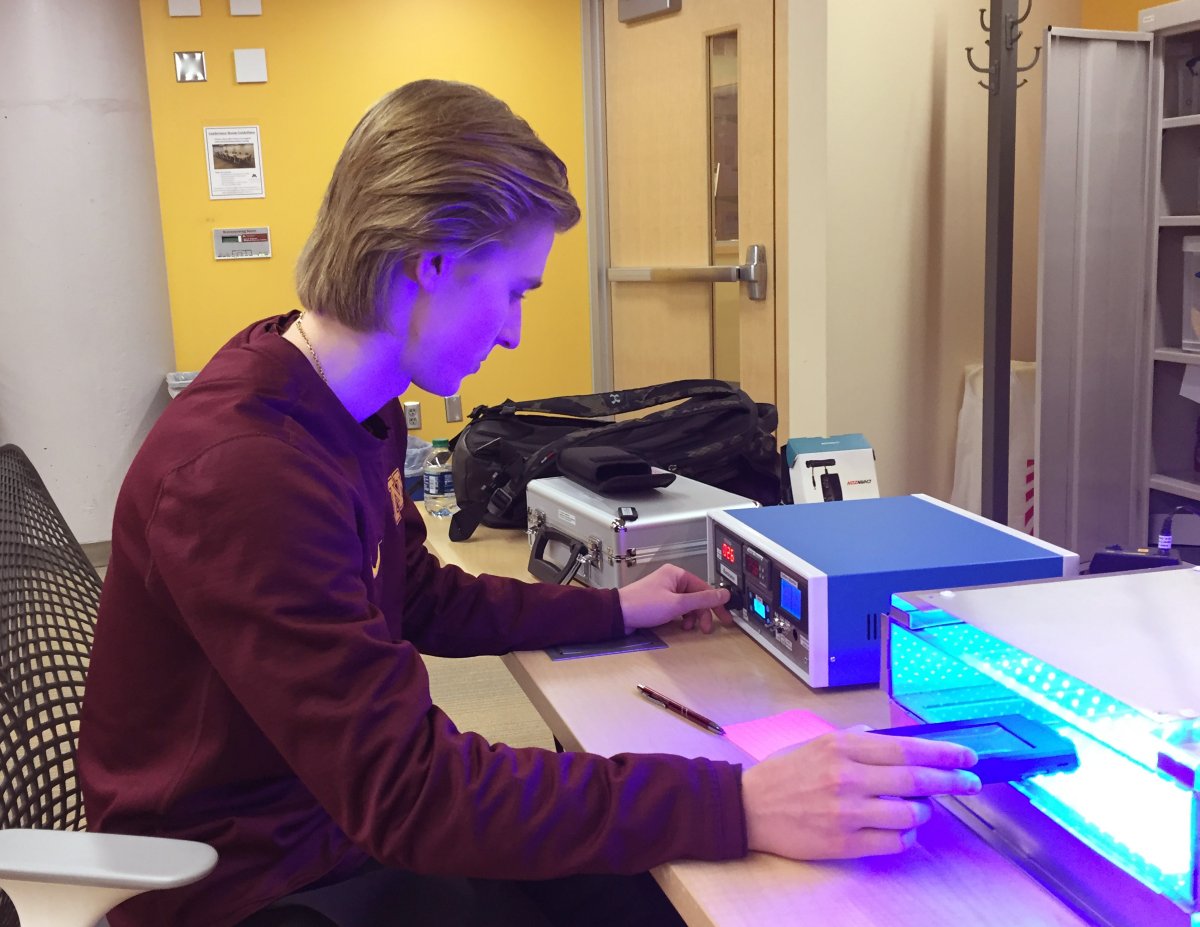
Bottom View Showing Optical Stack & Electronics...
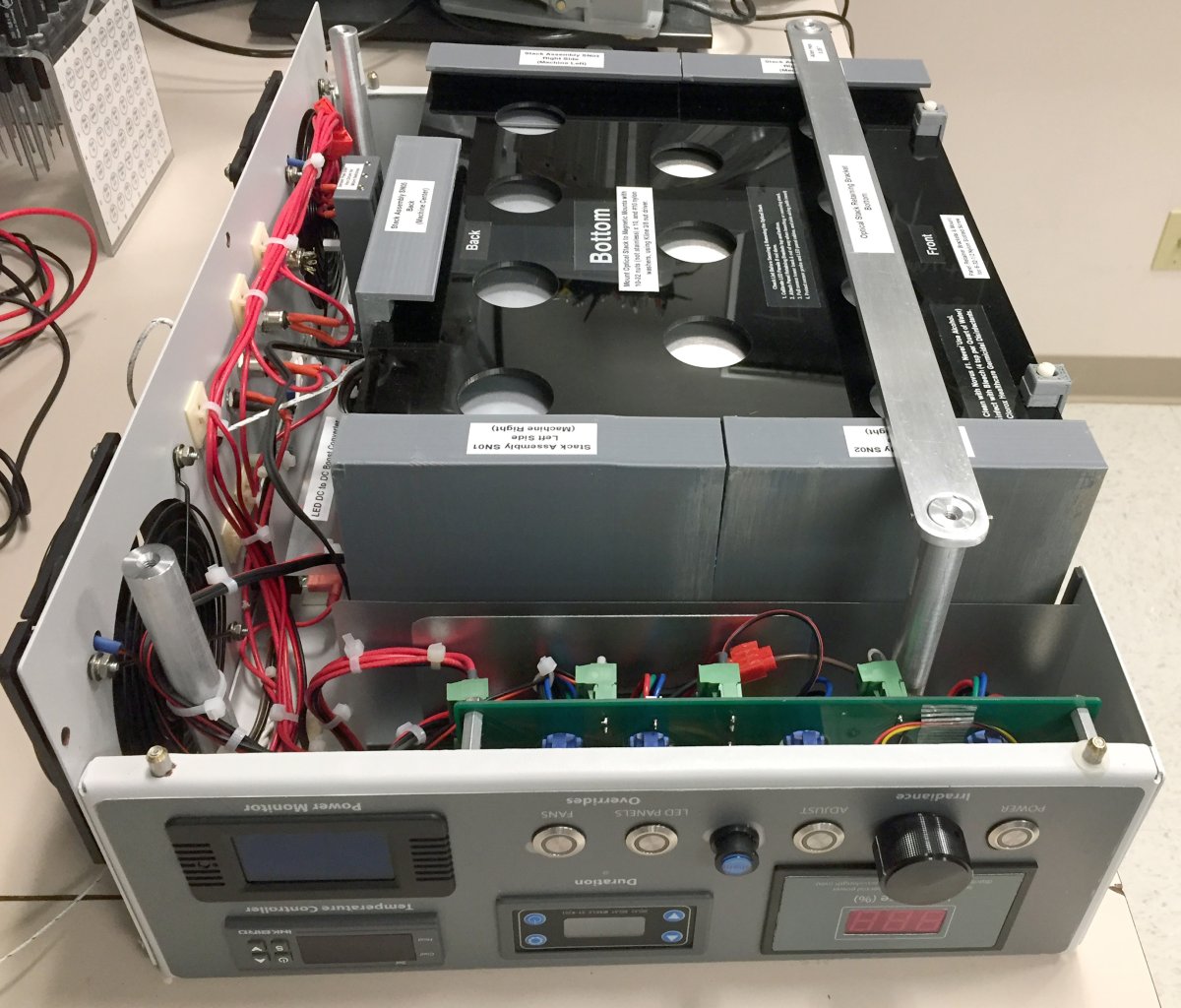
Integration of the removable optical stack, electronics and control console are all visible in this bottom view of the final instrument.
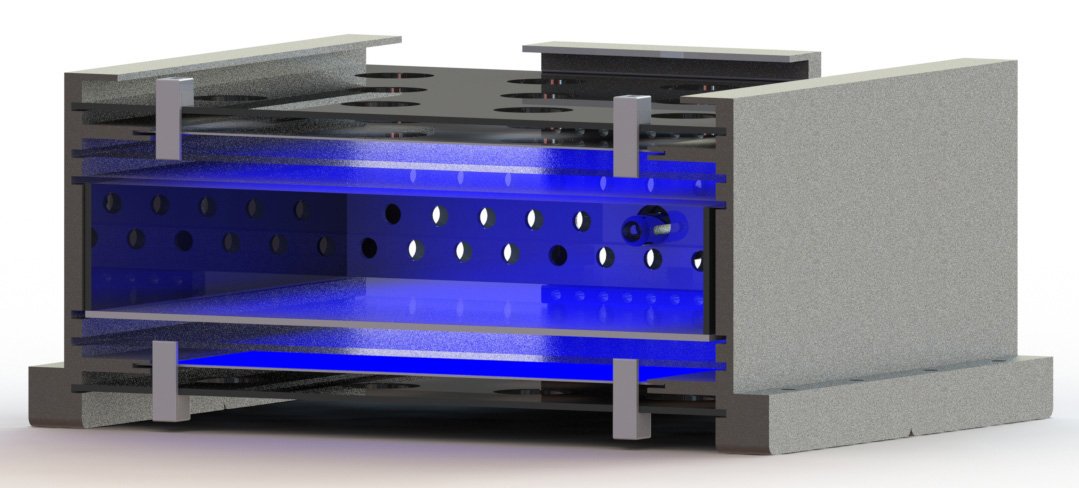
Optical Stack Subassembly with Clear Hand Compartment...
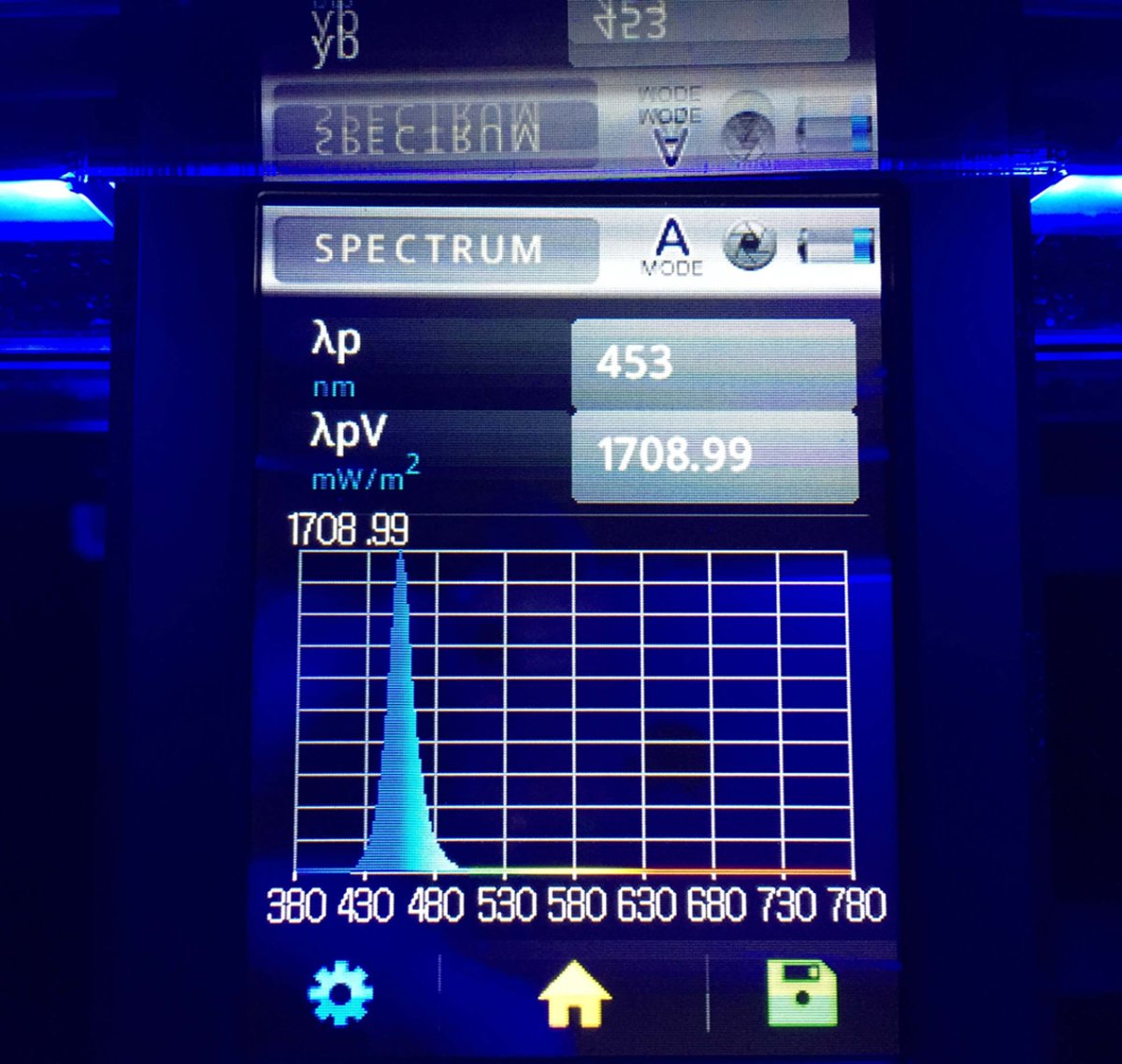
Custom LED Panel Calibration Assist Device...